Artemilife
ArtemiLife is a pioneering health and wellness company exploring the therapeutic potential of Artemisia annua (AA). Based in Kentucky, ArtemiLife cultivates AA crops and infuses the plant into daily-use products like coffee and tea. These products are currently being evaluated in human cancer clinical trials as potential maintenance therapies, including a completed Phase 1 trial in ovarian cancer and an ongoing Phase 2 trial in prostate cancer.
Pioneering
Health & Wellness
A Journey of innovation
a real-time discovery process
From day one of this six-year journey, Mighty was engaged as a fully integrated partner. The project has unfolded as a real-time discovery process—part innovation lab, part R&D expedition—revealing how the AA plant could be transformed into a functional product with both consumer and clinical potential.
Our mandate was to help bring an idea to life, from seed to shelf. That began on the ground—literally—traveling to farms outside Lexington, KY, working directly with agricultural experts to understand AA cultivation and harvesting.
We built a fully integrated product development pipeline, including:
Sourcing and coordinating raw material shipments
Overseeing formulation, testing, and production
Designing packaging and consumer formats suitable for both trial patients and general consumers

from seed to shelf
Mighty also led brand development, including visual identity, tone of voice, product literature, website design, and ongoing brand evolution as trial data and regulatory claims progressed.
We manage ArtemiLife’s marketing ecosystem, encompassing sales support, social media strategy, and all direct-to-consumer and B2B communications.
Supporting Clinical Trials
these clinical trials are a big deal for cancer research
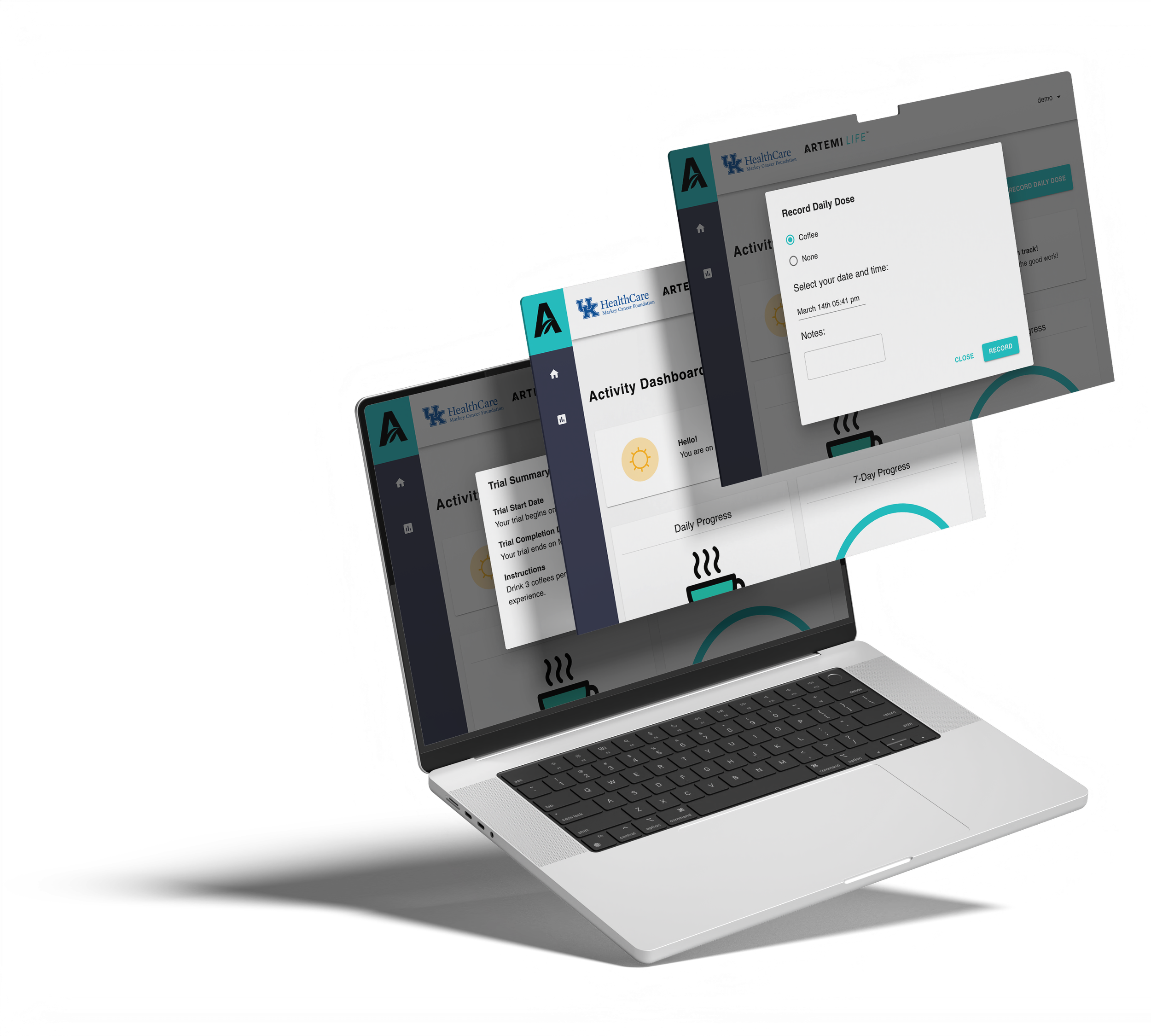
ArtemiCafe Decaf, the coffee product of ArtemiLife, was studied in clinical trials to prove its effectiveness in patients with ovarian cancer. To bridge the gap between patients and clinicians, we also developed a custom web app that enables cancer patients to track their daily ArtemiCafé intake, replacing traditional paper logs with real-time digital tracking. The tool provides instant accountability and seamless communication for healthcare teams overseeing these trials.
With Mighty’s strategic and operational support, ArtemiLife has completed a Phase 1 ovarian cancer trial and is progressing through a Phase 2 prostate cancer trial. As the product advances through the FDA’s Investigational New Drug (IND) process, we continue to evolve the brand, expand visibility, and prepare for large-scale market adoption.
Mighty remains a long-term partner, guiding ArtemiLife across brand, marketing, digital innovation, and product development as they transition from a clinical niche to global consumer relevance.